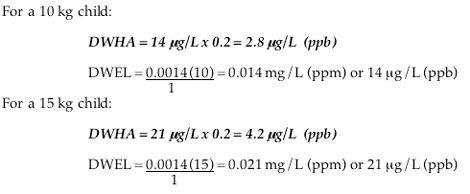A Critique of Community Briefings by The West Virginia Department of Environmental Protection on the Hazards of Drinking Water Contaminated With C8 (perfluorooctanoic acid — PFOA)
Kristina Thayer, Ph.D.
Throughout the spring and summer of 2002, the West Virginia Department of Environmental Protection (DEP) conducted a series of community meetings on the hazards of drinking tap water contaminated with perfluorooctanoic acid (PFOA, or C8) from DuPont’s manufacturing operations in Washington, West Virginia. Our analysis of the content of the materials presented by the DEP at these meetings finds them to be in conflict with positions of the U.S. Environmental Protection Agency (U.S. EPA), with studies in peer–reviewed scientific literature, and with industry–sponsored toxicity studies.
The following analysis refers specifically to statements made, and slides used in a presentation made by DEP staff and expert consultants on May 15 and 16, 2002. In describing the health concerns associated with C8 and the safety standards devised by the State, the DEP:
- Clearly indicates that C8 does not cause developmental and reproductive effects or present a long–term risk to people who drink it in their tap water. This contention is not supported by industry studies of C8 toxicity and is contradicted by high–ranking officials at U.S. EPA.
- Claims that tumors caused by C8 in animals are irrelevant to humans. The U.S. EPA considers C8 to be carcinogenic — causing liver, pancreatic, testicular, and mammary gland tumors [U.S. Environmental Protection Agency (U.S. EPA) 2002b, p. 6]. In company documents, DuPont scientists assert that the testicular and pancreatic tumors found in animals could be relevant to humans.
- Fails to account for documented food and air exposures to C8 when setting the drinking water contaminant limit, and then fails to tell the public of this omission, even though it constitutes a major deviation from standard US EPA protocols. DEP’s method allows between 15 and 100 times more C8 in tap water than would be allowed applying assumptions which account for other sources of exposure to C8 and include protections for the fetus, infants and children from the effects of suspected developmental toxins like C8 [Ammonium perfluorooctanoate (C8) assessment of toxicity team (CATT) 2002, p. 34; Appendix A].
- Asserts that the allowable amount of C8 contamination in drinking water is between 10 and 100 times more protective than it actually is. Had DEP applied the safety factors described at the community meetings, the screening level for C8 in drinking water would be between 1.5 and 15 parts per billion (ppb), not 150 ppb.
- Claims that the “safe” dose of C8 (the reference dose, or RfD) is five times more protective than that for the average chemical. It is not. The reference dose for C8 is five times lower than that for the average chemical because C8 is five times more toxic than the average chemical. The amount of protection provided by the C8 reference dose is equivalent to, or less than (see below) that provided for the average compound.
C8 and developmental/reproductive toxicity
On May 15th or 16th, 2002, DEP staff presented the following information to the public [West Virginia Department of Environmental Protection (WVDEP) 2002b, Slide #3]:
How toxic is C8?
• Not developmentally toxic
• Not a reproductive toxicant
[Excerpt | Full document]
Far from considering C8 “not developmentally toxic,” the U.S. EPA has initiated a “priority review” of C8 to determine whether expedited regulatory action against the compound is warranted under Section 4(f) of the Toxic Substances Control Act (TSCA) due to concerns that the compound causes adverse developmental and reproductive effects in animals.
As characterized by Oscar Hernandez, the director of the U.S. EPA’s Office of Prevention, Pesticides and Toxic Substances Risk Assessment Division: “Toxicological studies in rodents and primates have shown that exposure to PFOA (C8) can result in a variety of effects including developmental/reproductive toxicity, liver toxicity, and cancer” (U.S. EPA 2002c, p. 2:) [Excerpt | Full document].
According to Charles Auer, Director of the U.S. EPA’s Office of Toxic Substances, the agency is taking this action because it has received data suggesting that C8 has “a potential for reproductive/developmental toxicity,” and because “additional blood sample analysis data indicate low level exposures to the general population that are unexplained at this time” (U.S. EPA 2002d, p. 1) [Excerpt | Full document]
Our review of the studies available in the scientific literature shows that these findings are not new, and were available to the DEP well in advance of its public presentations.
For example, C8 causes numerous effects on male and female reproductive organs, such as testicular tumors (Biegel et al. 2001; Sibinski 1987), decreased sperm production (Sibinski 1987), mammary gland tumors (Sibinski 1987) and cellular effects in the ovary (Sibinski 1987). C8 also affects the prostate gland (York 2002) and increases estrogen levels (Biegel et al. 2001). In addition, C8 causes a significant increase in low birth weight pups in animal studies. Low birth weight is recognized as a risk factor for insulin resistance or Type II diabetes, high blood pressure, and cardiovascular disease later in life (Godfrey and Barker 2001, Hales and Barker 2001). Studies have also shown that health risks remain even for those low–birth weight children who achieve normal weight later in childhood, including risks for high blood pressure, stroke, insulin resistance and glucose intolerance (Eriksson et al. 2000a; Eriksson et al. 2002; Eriksson et al. 2000b; Eriksson et al. 1999; Eriksson and Forsen 2002; Forsen et al. 2000; Ong and Dunger 2002; Stettler et al. 2002).
More recent work shows that C8 significantly increases mortality in developmentally–exposed rats at a dose that did not affect parent survival (York 2002). These findings suggest that fetuses, infants and children may be more sensitive to C8 exposure than adults. Additional effects observed in developmentally–exposed male rats – at the lowest doses tested – include decreased body weight; increased liver, seminal vesicle (a part of the male reproductive tract), and kidney weight; and decreased weight of the spleen, an important organ in the immune system. At higher doses, C8 delayed sexual maturation of male and female rat pups (York 2002).
C8, cancer, and other long–term health risks
Also, in Slide #3 from DEP’s public presentation on May 15 or 16, 2002, DEP staff incorrectly represent the relevance to humans of C8 cancer studies in laboratory animals:
How toxic is C8?
• Cancer — rodents; mechanism not relevant to humans
[Excerpt | Full document]
DEP implies that all types of tumors (liver, mammary gland, testicular and pancreatic) caused by C8 in rodents are not relevant to humans. This statement is not supported by the science, nor has any regulatory body concluded similarly. Indeed, not even DuPont or 3M have attempted such a sweeping denial of C8's health risk to humans.
The manufacturers have argued that one of the mechanisms of action for liver tumors in rats is a cellular process called peroxisome proliferation, a mechanism generally thought to be much less relevant for humans than for rodents. Recent studies in primates, however, showed liver toxicity for C8 without peroxisome proliferation. Primates are similar to humans in liver physiology and function. Specifically, C8 has been found to cause death in monkeys along with liver and gastrointestinal tract toxicity, increased triglycerides levels and transient decreases in thyroid hormone even though “there was no evidence of peroxisome proliferation... suggesting a different mode of action than observed in rats” (U.S. EPA 2002b, p.5, 53–56) [Excerpt | Full document]. In fact, the lowest dose of C8 administered to monkeys caused “liver toxicity and possible mortality” (U.S. EPA 2002b, p.56) [Excerpt | Full document].
3M and DuPont failed to persuade the European Union with this same peroxisome proliferation argument when attempting to refute liver tumors caused by the chemical cousin to C8, known as PFOS. The EU review, which is the most definitive review of PFOS toxicity to date, concluded "there was no evidence of peroxisome proliferation in the livers of the treated animals” – even though these animals had liver tumors (Organisation for Economic Co–operation and Development 2002, p. 7) [Excerpt | Full document] – indicating that liver tumors could be induced by a different mechanism that may well be relevant to humans.
According to the DEP's Dr. Staats in her May 15, 2002 presentation at Blennerhassett Jr. High School, one of the impetuses for the DEP investigation of C8 stems from the “toxicity of similar compound PFOS” (WVDEP 2002c, slide #3) [Excerpt | Full document], a chemical that 3M phased out of production this year, under pressure from U.S. EPA, when studies showed widespread human exposure and fetal deaths in low–dose animal studies.
Regardless of how the arguments for the C8–induced liver tumors evolve, C8 has also been shown to cause tumors in the mammary gland, testes, and pancreas (Biegel et al. 2001; Sibinski 1987; U.S. EPA 2002b, p.6). Scientists at U.S. EPA have not concluded that a mechanism unique to rodents is responsible for all of these tumors (U.S. EPA 2002b, p. 75–78). Even DuPont found that for testicular and pancreatic tumors, "the mechanism of tumorigenesis is not completely understood, and therefore the relevance to humans can not be completely ruled out" (DuPont 1997, p.25) [Excerpt | Full document].
DEP’s “safe” C8 level in tap water is based on an ad-hoc methodology that allows far more C8 in tap water than the standard assumptions used by the U.S. EPA.
In a May 9, 2002 press statement, DEP writes:
“The team determined that a water level that contained less than 150 parts per billion of C8 would not cause harm to humans,” said Dr. Dee Ann Staats, West Virginia Department of Environmental Protection science adviser.
“The team thoroughly reviewed the scientific studies and unanimously agreed to the human health protective levels for drinking water,” Staats said. “We brought together some of the best scientists in the country. I am confident the human health protective levels established by the team are supported by the data.” (WVDEP 2002d)
[Read full press release on DuPont's website]
What the DEP didn’t tell the public is that in setting this standard, the State deviated from its draft assessment and from standard assumptions used by the U.S. EPA when setting contaminant standards for drinking water. The effect of these omissions was to permit substantially more C8 in tap water than would be allowed using standard assumptions.
First, the WVDEP stripped an important safety factor from its final calculations of a drinking water safety limit, tripling the allowable level of C8. WVDEP initially used this safety factor in draft calculations to account for some of C8’s unusual properties, including its long half-life in the human body and its persistence in the environment (WVDEP 2001) [Excerpt | Full Document]. C8 is not known to ever break down in water, soil, air, or the human body, a unique property that heightens public health concerns and that merited an additional safety factor in the department’s draft calculations. Not only did DEP staff strip this safety factor from final calculations, but also they then presented materials to the public implying that the safety factor had been left in place [Excerpt | Full document]. If this were true, the allowable level of C8 in drinking water would be 50 ppb, not 150 ppb.
Second, the state ignores potentially substantial background exposures to C8 from contamination in air and food. Water is not the only – and may not be the primary – route of exposure to C8. 3M reports that PFOA is present in green beans, apples, bread, and ground beef at concentrations comparable to what has been found in drinking water supplies around DuPont’s Washington Works plant (U.S. EPA 2001b, p. 1) [Excerpt | Full Document]. Recent modeling of C8 concentrations conducted by DuPont show significant levels of C8 in the air, especially northeast of the Washington Works Plant across the Ohio River, exceeding safety limits set by the WVDEP (DuPont 2002).
For contaminants with multiple routes of exposure, U.S. EPA’s standard assumption is that 20 percent of contaminant comes from drinking water, and 80 percent comes from other sources (EPA 1992). Had DEP used this standard assumption, allowable amount of C8 in tap water for an adult would be closer to 30 ppb, not 150 (Appendix A). Adding back in the Department’s safety factor for persistence would bring the drinking water screening level to protect adults down to 10 ppb.
In addition, the Department fails to set a protective level for infant exposures. Specifically, WVDEP did not account for the significant body weight difference between adults and infants. Bottle–fed infants fed formula reconstituted with C8-contaminated tap water are a population of high concern. Bottle-fed infants receive the highest dose of all drinking water contaminants, pound for pound, of any other segment of the population. This is because when an adult and an infant drink a liter of contaminated water, C8 will be more concentrated in the child — resulting in a higher dose per unit of body weight.
A C8 level protective of bottle-fed infants would incorporate three modifying factors WVDEP has ignored – a correction for infant body weight, a safety factor for C8 persistence, and a factor to account for multiple routes of exposure (such as air). Incorporating all three factors gives an allowable level for C8 in drinking water of 1.5 ppb (Appendix A), well within the range of C8 levels found in the local drinking water sources. For instance, the peak C8 levels in springs that provide drinking water within one mile of the Washington Works facility were above 10 ppb (WVDEP 2002a, Slide 8) [Excerpt | Full Document]. Average C8 levels in this same water were 4 parts per billion. During the early 1990’s, DuPont established its own Community Exposure Guideline (CEG) for C8 in drinking water at 1 ppb (CATT 2002, p. 46) [Excerpt | Full Document].
Considering that infants may be more vulnerable to C8, the standard may need to be more conservative than what we have outlined here. Animal studies show that C8 is more toxic to newborns; for example, it causes mortality in young rats at doses that did not kill parent animals. Studies also show that the highest blood concentrations of C8 in non–worker populations occur in children (U.S. EPA 2002b, p. 3) [Excerpt | Full Document] reinforcing the need for DEP to lower the C8 safety limits to protect infants.
DEP implies that tap water safety limits of C8 are 10 to 100 times more protective than they actually are.
In another public meeting presentation, on either May 15 or May 16, 2002, DEP staff imply that they applied a large safety buffer when deriving the drinking water safety limit for C8 (WVDEP 2002b, Slide 5) :
How are safe levels developed?
No Observed Effect Level (NOEL) typically divided by at least 1,000 up to 10,000 Safety Factor
• Animals to humans
• Sensitive subpopulations
• Chronic lifetime exposure
• Other criteria such as biopersistence
[Excerpt | Full document]
The problem here is that the DEP did not apply the “typical” safety margin of 10,000 — or even 1000. Instead, DEP applied a safety factor of only 100 to account for possible differences between small groups of rats in the laboratory and large populations of humans exposed through contaminated drinking water. Also, some of the listed criteria, such as biopersistence, were not taken into account at all. The most recent data from 3M shows that it takes humans 4.4 years to get rid of just half the amount of C8 in the body after all C8 exposures end (U.S. EPA 2002a, p. 2) [Excerpt | Full document]. If persistence were including when determining a safe dose of C8, the allowable amounts would have been much smaller, in part because there is no reason to believe the C8 exposures are ending.
DEP states, wrongly, that the standard for C8 in drinking water is somehow more protective than contaminant limits applied to other chemicals.
During a May 15, 2002 public meeting at Blennerhassett Jr. High School, DEP staff presented the following slide:
How does the C8 RfDo (reference dose, which means the daily “safe” dose) compare to that for other chemicals?
The average EPA–developed oral RfD is 0.02 mg/kg–day which is 5 times higher than C8’s of 0.004 mg/kg–d. Therefore, C8’s risk factor is more conservative or health protective than that for the average chemical. (WVDEP 2002c, Slide #15)
[Excerpt | Full document]
C8’s reference dose is not more health protective than that for the average chemical. C8 carries a lower reference dose than the average chemical because it is more toxic than the average chemical, approximately 5 times more toxic, according to DEP’s own calculations. C8’s low reference dose simply reflects this difference.
In truth, far from being more protective than average, there are several reasons why the DEP risk factor may not be sufficiently protective. First, a dose that causes no observed adverse effect level, or a NOAEL, was not identified in two critical studies, the cancer study, and the reproduction study. The reproduction study was used by the DEP-led team to set a RfD for C8. Typically, toxicologists design animal studies with the expectation that the lowest dose tested will not produce adverse effects in laboratory animals. C8’s manufacturers underestimated the toxicity of C8, and missed the no effect level in both of these studies — even the lowest doses tested caused observable adverse effects. It is not at all accurate to characterize a risk factor derived from studies that found adverse effects at all doses as “more conservative or health protective” than the average risk factor.
Second, the study used to derive the RfD (a two–generation reproductive toxicity study) is not a chronic study, and is not truly indicative of the hazards of long term consumption of C8 in tap water. In this study, animals exposed in utero are followed, at a maximum, until they are young adults (about 4 months). This design makes it impossible to address questions such as whether in utero or early life exposure to C8 can predispose an individual to cancer, degenerative nervous system disorders, diabetes, or other diseases more prevalent at the end of life. Third, C8 is an extremely biopersistent chemical, which was not taken into account in setting the reference dose (see above). Biopersistence is a signature characteristic of C8, and must be explicitly considered in determining chronic risks faced by consumers of C8.
Finally, the RfD assumes that the control animals in the critical studies (those not directly administered C8 in the study) had no exposure to C8. This is not true. Control animals have been consistently shown to have significant liver or serum levels of C8 (U.S. EPA 2002b, p. 48, 57–60) [Full document]. The fact that the RfD is based on studies with no true controls means that important toxic effects may have been overlooked because they were also caused by C8 in the contaminated control animals.
Conclusions
On September 27, U.S. EPA initiated a priority review of C8 because of their concerns about its developmental and reproductive effects, and because “additional blood sample analysis data indicate low level exposures to the general population that are unexplained at this time” (U.S. EPA 2002d, p. 1) [Excerpt | Full document].
The EPA’s concerns focus on exposures in the general population, among people who face lower overall potential for exposure than do the West Virginia communities whose drinking water supplies have been directly contaminated with C8 from manufacturing and disposal operations. The studies that instigated U.S. EPA’s priority review were available to DEP well in advance of its public presentations.
In light of the federal government’s position on the potential for C8 to harm reproduction and development, and its potential to cause cancer in humans, it appears that DEP’s interpretation of C8’s toxicity, and the drinking water safety limits derived from that interpretation, fail to give the affected communities an adequate margin of protection.
APPENDIX A
WVDEP (C8 Assessment of Toxicity Team, or CATT) APPROACH:
The approach used by WVDEP to calculate a drinking water screening level for C8 is outlined below.
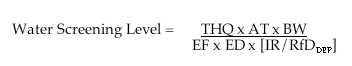
THQ = Target Hazard Quotient, assumed to be 1
RfDDEP = The oral reference dose estimated by DEP, 0.0042 (truncated to 0.004) mg/kg–day
BW = Body weight, assumed to by 70 kg for adults (children are not considered in DEP calculations)
AT = Averaging time, 10950 days, the exposure duration expressed in days
EF = Exposure frequency, 350 days/year, the average number of days each year people are exposed
ED = Exposure duration, 30 years, the average number of years people are exposed
IR = Ingestion rate, assumed to be 2 L/day for adults (children are not considered in DEP calculations)
So,
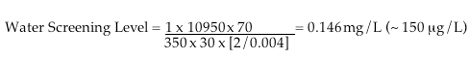
U.S. EPA APPROACH:
The standard U.S. EPA approach for developing drinking water safety limits is presented below. U.S. EPA incorporates a factor called the Relative Source Contribution in cases of chemicals like C8 associated with multiple routes of exposure. Calculations below also reflect the safety factor of 3 that DEP stripped from its final calculations that were originally in place to account for C8's persistence and long half-life in the human body. Both of these chemical characteristics contributed to U.S. EPA's decision to conduct a priority review of C8.
Adult Lifetime Drinking Water Health Advisory (DWHA):
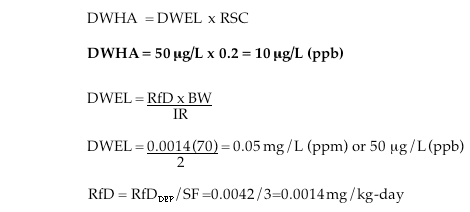
DWHA = Drinking Water Health Advisory; “safe” dose of C8 via drinking water
DWEL = Drinking Water Equivalent Level; conversion of reference dose to reference concentration
RfD = The oral reference dose that accounts for persistence and long half-life in the human body
BW = Body weight, assumed to by 70 kg for adults, 10 – 15 kg for children and 5.2 for bottle fed kids
IR = Water ingestion rate; 2 L/day for adults and 1 L for children and bottle–fed infants.
RSC = Relative Source Contribution, which has a U.S. EPA default value of 20% (0.2)
SF = safety factor to account for persistence and long half-life in the human body (3X)
Bottle–fed Infant Drinking Water Health Advisory (DWHA):
In this case, we assume that a bottle–fed infant weight 5.2 kg and drinks 1 L /day, consistent with the scientific literature and U.S. Department of Agriculture formula consumption data.
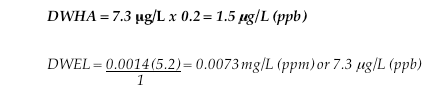
Drinking Water Health Advisory for a 10 or 15 kg child (DWHA):
For comparison against the limit derived for an exclusively bottle-fed infant, limits protective of a 10 or 15 kilogram child are presented below.